
University Presentation Showcase: Graduate Poster Gallery
Preview
Creation Date
Spring 2017
Major
Chemistry
Department
Chemistry
Degree
Graduate
Mentor
Donghui Quan
Mentor Department
Chemistry
Abstract
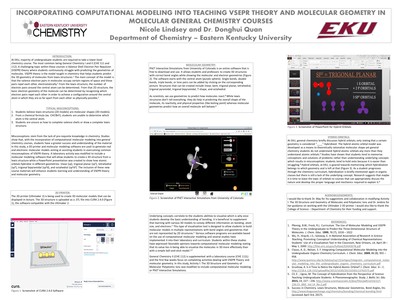
At EKU, majority of undergraduate students are required to take a lower-level chemistry course. The most common being General Chemistry I and II (CHE 111 and 112). A challenging topic within these courses is Valence Shell Electron Pair (VSEPR) theory where students continuously struggle with predicting the geometries of molecules. A common misconception among the students is predicting the 3D structure from a given lewis structure and it stems from students lacking the pre-requisite knowledge of the material. By incorporating technology of computational molecular modeling into these courses, studies show that students have a greater success and better understanding of the material. In this study, a 3D printer and molecular modeling software are used to generate real and electronic molecular models aiming at assisting the students in overcoming misconceptions of VSEPR theory. A laboratory activity was also modified to include a molecular modeling software that allowed students to create a 3D structure from a lewis structure while a PowerPoint presentation was created to show how atomic orbitals hybridize in different geometries. Inclusion of all these new course materials will enhance students learning and enrich their understanding on the molecular geometry.

